Published on
Gene therapy treating the neurodegenerative disease, SMARD1, shows promising results in mice studies.
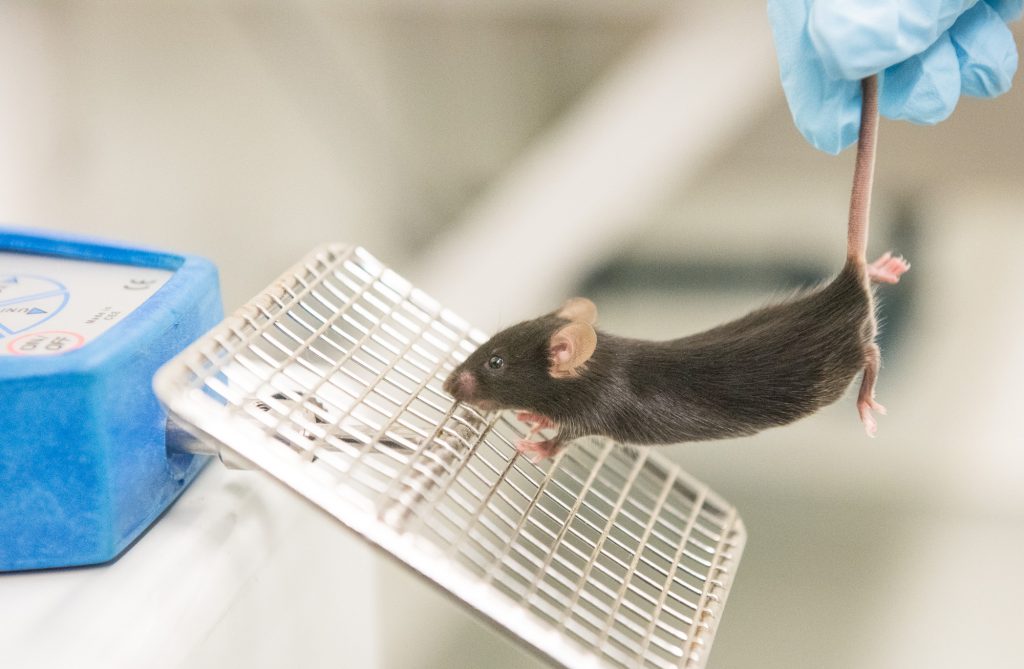
Shababi uses an instrument to measure grip strength in the forelimbs of mice. Healthy mice are able to cling on with a stronger grip than SMARD1 mice. | photo by Jennifer Lu, Bond LSC
Monir Shababi was confident her experiments treating a rare genetic disease would yield positive results before she even ran them.
Scientists had success with a similar degenerative neuromuscular disease, so she had every expectation their strategy would work just as well in her mice.

Monir Shababi, an assistant research professor in the Department of Veterinary Pathobiology, studies SMARD1 in mice. | photo courtesy of the Department of Veterinary Pathobiology
“I was expecting to get the same results,” said Shababi, an assistant research profession in Christian Lorson’s lab at the University of Missouri Bond Life Sciences Center. Shababi studies spinal muscular atrophy with respiratory disease type 1, or SMARD1.
The treatment worked, but not without a few surprises.
Her findings, published in Molecular Therapy, a journal by Nature Publishing Group, are one of the first to show how gene therapy can effectively reverse SMARD1 symptoms in mice.
In patients, SMARD1 is considered such a rare genetic disorder by the U.S. National Library of Medicine that no one knows how frequently the disease occurs. It’s only when babies develop the first symptom—trouble breathing–that pediatricians screen for SMARD1.
Shortly after diagnosis, muscle weakness appears in the hands and feet before spreading inwards to the rest of the body. The average life expectancy for a child diagnosed with SMARD1 is 13 months. There is currently no effective treatment.
Since the neuromuscular disease is caused by a recessive gene, SMARD1 comes as a shock to the parents, who are carriers but do not show signs of the illness, Shababi said. This genetic defect prevents cells from making a particular protein that scientists suspect is vital to replication and protein production.
The hereditary nature of the disease has a silver lining, though. Because SMARD1 is a caused by a single pair of faulty genes and not multiple ones, it is a prime candidate for gene therapy that could restore the missing protein and reverse the disease.
To do that, Shababi set up a dose-response study using a tiny virus to carry the genetic instructions for making the missing protein. She injected newborn mice with a low dose of the virus, a high dose, or a placebo with no virus at all.
Injecting at different doses allowed her to ask which dose worked better, Shababi said.
According to the previous research, a higher dose should have resulted in a more effective treatment.
“So I thought a higher dose was going to work better,” Shababi said.
Instead, the high dose had a toxic effect. Mice given more of the virus died sooner than untreated mice. Meanwhile, mice given a low dose of the gene therapy lived longest. They regained muscle function and strength in both the forearms and the hind limbs and became more active.
In fact, some of them survived long enough to mate and produce offspring.
Initially, Shababi housed her SMARD1 mice in the same cage as their mothers so that the moms could intervene if the sick pups become too feeble to feed themselves. When the male pups became well, their moms became pregnant.
“That was another surprise,” Shababi said. “That was when I knew I had to separate them.”
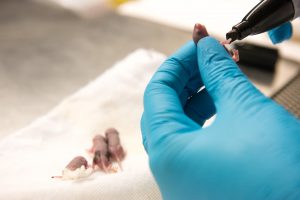
Shababi marks a pup, only a few days old, with permanent marker so each mouse in her study can be identified. | photo by Jennifer Lu, Bond LSC .
In another twist, Shababi discovered that the route of injection also mattered.
To get the treatment across the blood-brain barrier and to the spinal cord, Shababi used a special type of injection that passes through the skull and the ventricles of the brain, and into the spine.
This was no easy task.
The newborn mice were no larger than a gummy bear. To perform the delicate work, Shababi — who has written a chapter in a gene delivery textbook about this procedure — had to craft special needles with tips fine enough for this injection. She added food coloring to the injection solution so she could tell when it had reached its intended destination.
“After half an hour, you will see it in the spinal cord,” Shababi said. “The blue line in the spine: that’s how you can monitor the accuracy of the injection.”
Unfortunately, repeated injections in the mice caused hydrocephaly, or swelling in the brain.
“They get a dome-shaped head,” Shababi explained.
The swelling happened in all three treatment groups, but most frequently in the group that received a high dose of viral gene therapy. This reinforced the finding that while a low dose was beneficial, a high dose was even more harmful than no treatment at all. It’s unclear why.

Christian Lorson is a professor of veterinary pathobiology at the Bond LSC. His research focuses on spinal muscular atrophy and more recently, SMARD1. | photo by Hannah Baldwin, Bond LSC .
The Lorson lab plans to continue studying SMARD1 and this treatment, in particular, how changing the delivery routes for gene therapy can improve outcomes in treating SMARD1.
“It’s not as simple as replacing the gene,” Lorson said. “It comes down to the delivery.”
Injections in the brains of mice are meant to mimic spinal cord injections in humans, but intravenous delivery could be another option. However, intravenous injections, which travel through the blood stream and to the entire body, might cause off-target effects that could interfere with the effectiveness of the treatment.
Once researchers better understand how to optimize dosing and delivery on the cellular and organismal level, the therapy can move closer to clinical trials, Lorson said.
Even though gene therapy for SMARD1 is still in its early stages, he said he was optimistic that developing treatments for rare genetic diseases is no longer the impossible task it seemed even ten years ago.
Spinal muscular atrophy (SMA) is a prime example of a recent success, Lorson pointed out. In the last six years, gene therapy for that disease has moved from the research lab to Phase I clinical trials.
“While it feels like a long time for any patient and their families,” Lorson reassured, “things are moving at a breakneck pace.”
The study, “Rescue of a Mouse Model of Spinal Muscular Atrophy With Respiratory Distress Type 1 by AAV9-IGHMBP2 Is Dose Dependent,” was published in Molecular Therapy, a journal published by Nature Publishing Group. This work was supported by a MU Research Board Grant (C.L.L.); MU College of Veterinary Medicine Faculty Research Grant (M.S.); the SMA Foundation (C.P.K.); National Institute of Health/National Institute of Neurological Disorders and Stroke grants; and the Missouri Spinal Cord Injury Research Program (M.L.G.).