Published on
MU scientists develop model to study complex pregnancy disease
By Danielle Pycior | Bond LSC
Researchers have been exploring the complicated and difficult world of pluripotent stem cells for 15 long years on the second floor of the University of Missouri’s Bond Life Sciences Center.
A type of stem cell that can be turned into any cell in the human body and self-replicate, scientist Michael Roberts and his lab have used these cells to study the early stages of pregnancy in the hopes of understanding preeclampsia — the number one cause of maternal mortality worldwide.
Roberts and his lab have been using an innovative technique to see what occurs at the early stages of the disease with the ambition of understanding the disorder. Their most recent advancements were published in Proceedings of the National Academy of Sciences in March.
Their work has focused on the complex interaction between a mother’s body and the placenta. This temporary organ connects a fetus via an umbilical cord to the uterine wall of the mother to provide nutrients, gas exchange and waste elimination for the developing child.
“The very fact that you can remove the placenta to cure the patient suggests that this maternal condition has its origin in the placenta” said Roberts.
Preeclampsia comes in two main forms, early-onset starting around 20 weeks of pregnancy, and late-onset occurring after about 35 weeks. Symptoms can range from high blood pressure to headaches, fatigue and other forms of stress in the mom. Severe forms of preeclampsia are life-threatening for mother and child, and the only treatment is removing the placenta, which forces the mother to undergo cesarean section, invariably prematurely.
Researchers have struggled to understand this disease for multiple reasons. While the underlying defect is generally believed to be shallow formation of the placenta and failure of certain cells called trophoblasts to invade sufficiently deeply into the wall of the womb, early detection in the first trimester is almost impossible right now, and, by the time doctors can detect it, the placenta has faced so much damage that it is of little use to researchers.
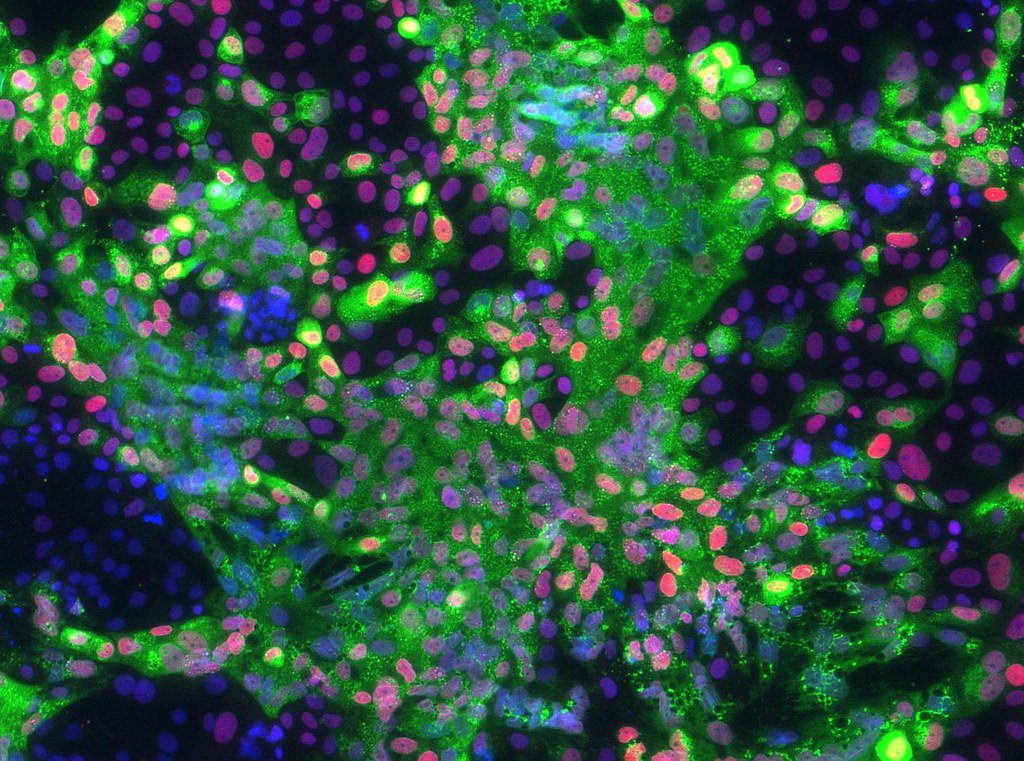
Working with University of Missouri Health Care physician Danny Schust and basic scientists Laura Schulz and Bond LSC’s Toshihiko Ezashi, Roberts’ lab acquired real-world samples of cells related to the disease. Mothers who came into MU Women’s and Children’s Hospital in Columbia diagnosed with early onset preeclampsia agreed to give part of the tissue from their umbilical cord after childbirth, which was then used to grow primary cultures of connective tissue cells. These were reprogrammed into stem cells that are essentially identical to embryonic stem cells. These induced pluripotent stem cells were then converted to trophoblast cells — the cells in the fetus that make up the outer layer of the placenta — and examined for abnormalities associated with the disease.
“If you do this successfully, you essentially recapitulate the early stages of the pregnancy that led to preeclampsia.” Roberts said.

After this, the researchers tested the cells under various conditions to try and figure out the early stages of the disorder, something that had been relatively impossible previously. Since preeclampsia is difficult to detect in early stages and early placentas from such patients are unavailable for ethical reasons, this approach has allowed researchers a unique look at what happens during a preeclamptic pregnancy.
They compared normal pregnancy cells with cells from preeclampsia pregnancies. By putting the cells in incubators, they observed them under different conditions and levels of stress. Under 5% oxygen, the cells acted similarly to the normal pregnancy, but under 20% oxygen, the cells were highly stressed, resulting in their inability to function properly.
“So, we essentially showed that at least one feature of these preeclampsia cell lines was that they weren’t able to cope with high oxygen and so invaded poorly,” Roberts said. “What we assume is that in the disease there is some sort of stress occurring that causes these cells to not behave properly.”
Roberts’ lab members Toshihiko Ezashi, a research professor at Bond LSC, and Megan Sheridan, a former MU graduate student in the Roberts’ lab who is now at University of Cambridge in England, discovered that there isn’t one gene that leads to preeclampsia, but a few genes within a large cohort of genes involved in controlling a trophoblast cell’s invasive properties.
While this discovery narrows the search for answers, it still leaves a lot of questions being asked.
“We are still nowhere near creating a test for preeclampsia and we are certainly far from a cure because how can you prevent a disease before you know a woman has it?” Roberts said.
This arduous, complicated and incredibly intriguing work looks ahead to dissecting the genetic abnormalities of the disorder in the hopes of creating a successful test and treatment, which could make all the difference for women worldwide.
“This is a disease that affects a large number of women,” Roberts said. “In this country, women can usually find proper care, but in countries outside the U.S. without hospitalization, the woman is going to die and so is the baby.”